Terminal coverage of domestic hospitals
7000
Third terminal
8900+HOUSEHOLDS
Pharmacy
4000+
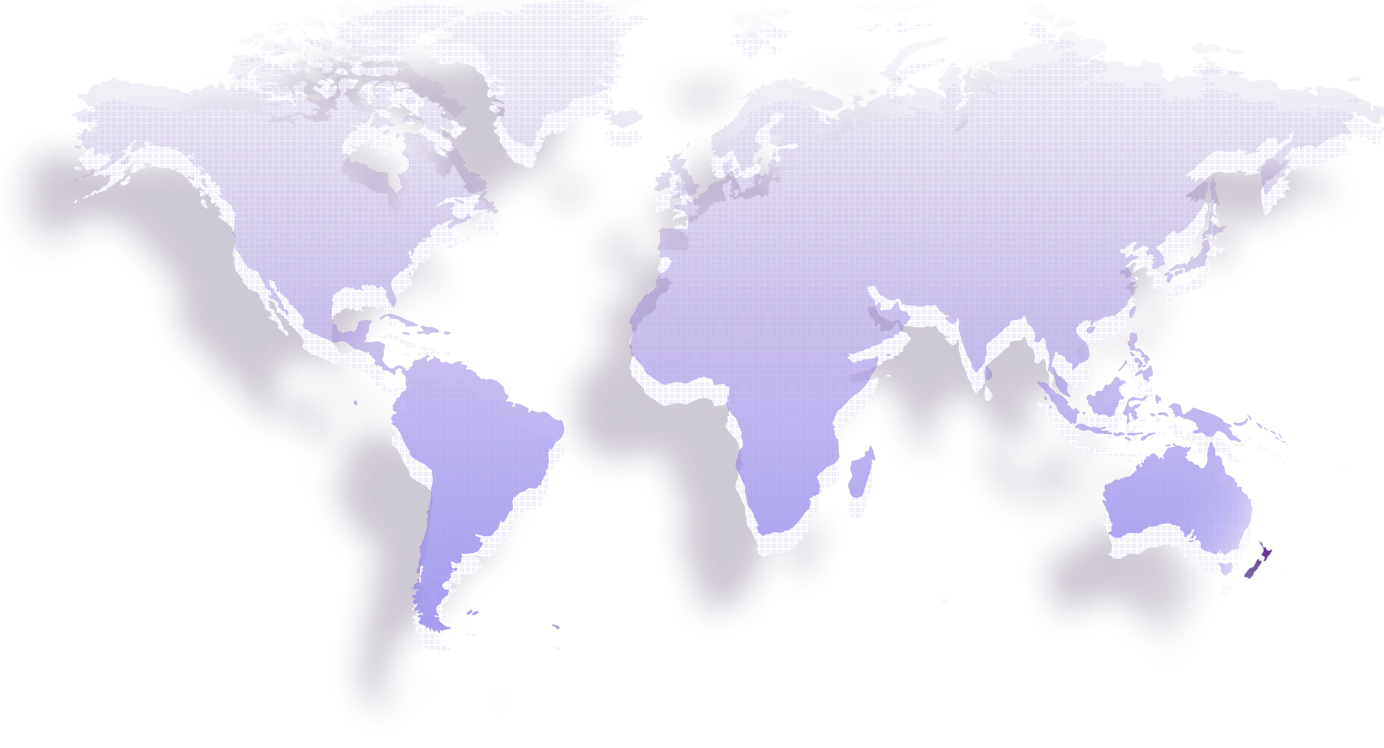
Go Global
20+years
Country/region
40+
Digital Dynamization
The quality system of Kexing Biopharm includes a quality control system, a quality assurance system, and a validation system.
Quality standards: To minimize the risks of contamination, cross contamination, confusion, and errors during drug production, and ensure the continuous and stable production of drugs that meet the intended use and registration requirements.
Quality policy: sound system, honesty and trustworthiness, safety and effectiveness, continuous improvement

R&D and innovation
Global R&D Center
R&D strategy: R&D of stratified medicines, serial medicines and progressive medicines according to R&D strategic planning with biologics as the core.
R&D and innovation
R&D field
R&D strategy: R&D of stratified medicines, serial medicines and progressive medicines according to R&D strategic planning with biologics as the core.
R&D and innovation
Animal Vaccine
R&D strategy: R&D of stratified medicines, serial medicines and progressive medicines according to R&D strategic planning with biologics as the core.
R&D and innovation
Synthetic Biology
R&D strategy: R&D of stratified medicines, serial medicines and progressive medicines according to R&D strategic planning with biologics as the core.
R&D and innovation
Bacteriophage technological platform
R&D strategy: R&D of stratified medicines, serial medicines and progressive medicines according to R&D strategic planning with biologics as the core.

Global R&D Center
R&D field
Animal Vaccine
Synthetic Biology

Bacteriophage technological platform
- News and information
- Video Center





 Jul 14,2025
Jul 14,2025